Can Oxygen Be Toxic?
Jun 01, 2019 • 166 views
I hope that you have read my previous article on scuba diving and enjoyed it.
In scuba diving world, ‘Oxygen Toxicity’ is a commonly referred word. When I was introduced to this term I was completely blown away. I was wondering how something that is essential to sustain life on earth can be toxic to human beings. After doing further study I realized that its quite an interesting topic. To start off, anything in excess is bad. Here’s what I learnt about oxygen toxicity:
Air is primarily composed of nitrogen (78%), oxygen (21%), and other gases (1%). This mixture works quite well on the surface. But our body doesn’t really care about the percentage of oxygen all it cares about the percentage pressure of oxygen (pO2). A healthy and fit individual can work perfectly between the pO2 range of 0.12 ATA and 1.6 ATA although the toxicity starts to act at 0.45 ATA. On the surface the pO2 is 0.2 ATA and with increase in every 33ft the pO2 increases by 0.2 and the atmospheric pressure increases by 1 ATA.
The association of depth and percentage pressure of oxygen is depicted below.
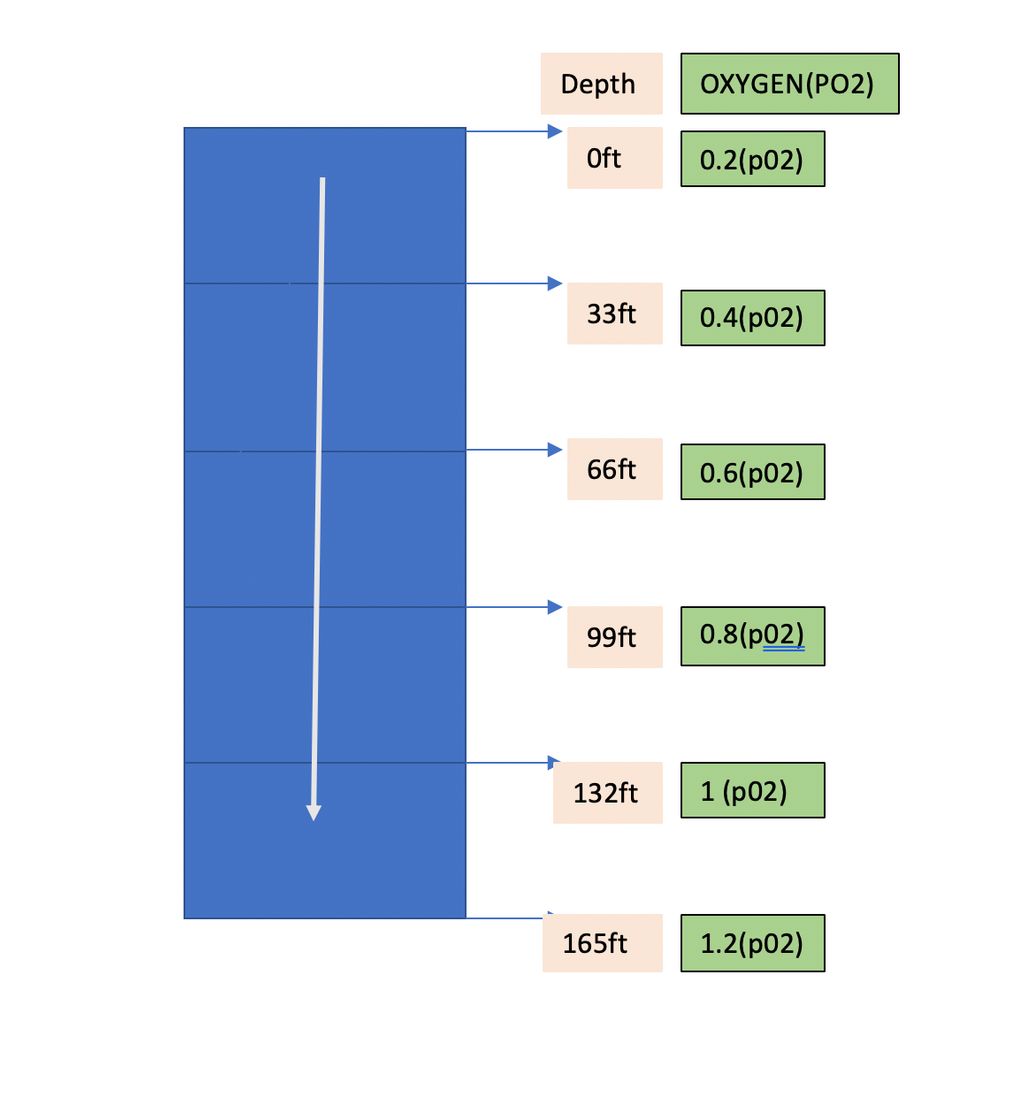
So, for a recreational diver, Oxygen Toxicity with normal air is not a big concern.
Then why am I bothered about it? It becomes relevant as the trouble is caused by its partner ‘Nitrogen’.
Although the normal air mixture works more or less fine for recreational dives, the large nitrogen content is the problem here. Divers face another issue called ‘nitrogen narcosis’. The exact causes of nitrogen narcosis hasn’t been discovered yet, but high amount of nitrogen in the blood affects the CNS (central nervous system) and gives a reversible narcotic effect.
As a diver goes down, he experiences increased pressure. As the pressure increases, more nitrogen gets dissolved in the blood stream. We have to release the nitrogen from the body, to avoid the narcosis, which necessitates the ascent and hence causing reduced dive time with increased depth.
To avoid this, a chunk of nitrogen is replaced by the oxygen (40%). This mixture is called NITROX, which allows divers to dive for a longer duration but at lower depth. The pO2 of nitrox at the surface is 0.4 ATA and we reach the dangerous limit of 1.6 ATA at a depth of 100ft with nitrox instead of 200ft with the normal mixture of air. So, Scuba diving has now become chemistry, physics and math of adjusting the gas, which they breathe based on various factors such as dive time, depth, pressure etc.
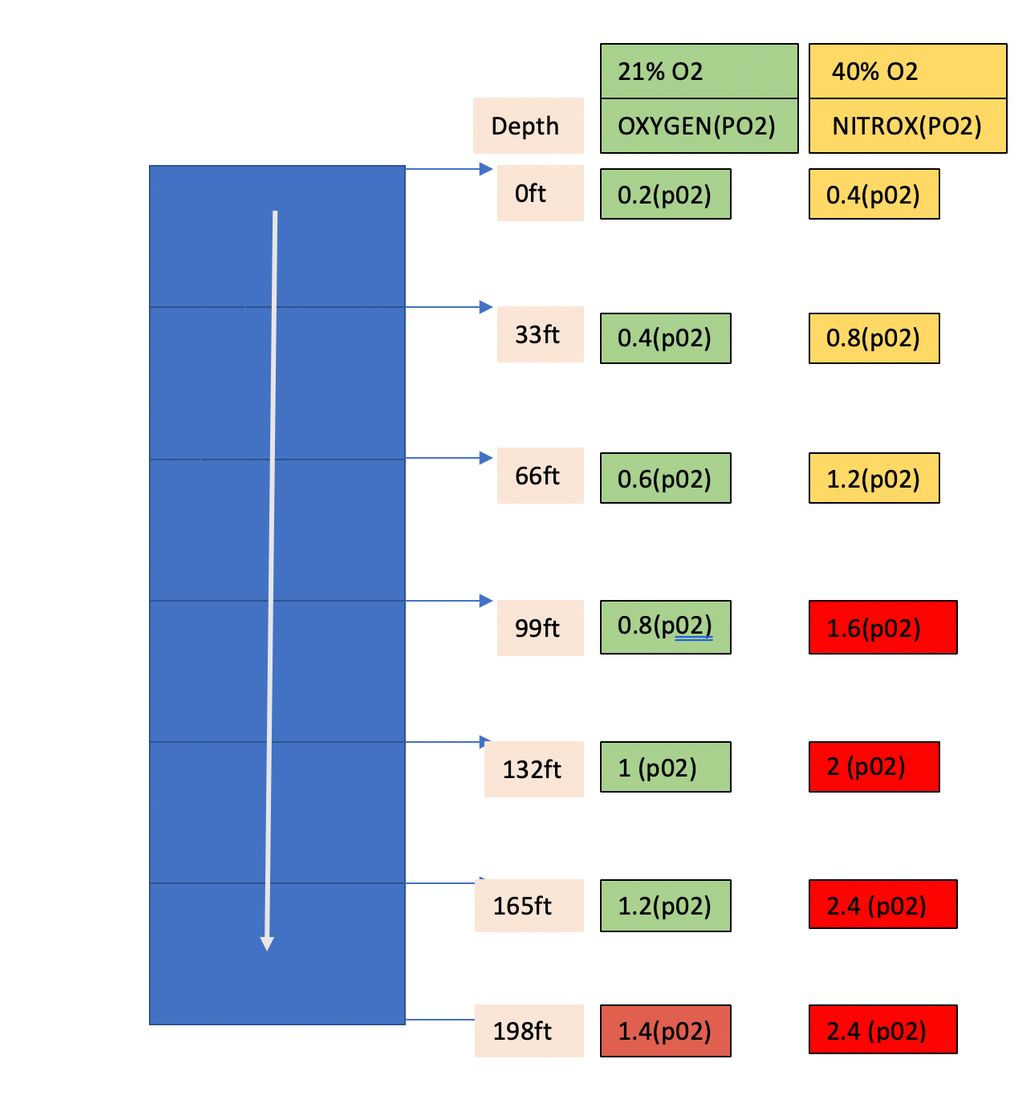
Now we are in a pickle. What does one have to do, to dive deeper and a longer duration?
Stay tuned for the answer.
